The Bond Order for a Single Covalent Bond Is
The difference between a single covalent bond or otherwise known as a single bond is that there is only sharing of one pair of electrons. Electronegativity is the tendency of an atom to attract electrons to another atom.

Covalent Bonding Lesson 1 Covalent Bonding Covalent Bonds Atoms Held Together By Sharing Electrons Mostly Formed Between Nonmetals Molecules Neutral Ppt Download
The overall bond order is all the bond orders added up to a grand total.

. Atoms come together to bond in order to. A covalent bond can also be a double bond or a triple bond. A single covalent bond is one in which two electrons are shared by two atoms in which one electron comes from each atom.
In a water molecule are held together by covalent bonds. A bond order of 1 means there is a single bond between two atoms A bond order of 2 means there is a double bond between two atoms. Bond number gives an indication of the stability of a bond.
Single Covalent Bond Double Covalent Bond Triple Covalent Bond. It is very straight forward - a single bond has a bond order of 1 a double bond has a bond order of 2 and a triple bond has a bond order of 3. The shared valence electrons between two nonmetal atoms is called a covalent bond.
What is the number of electrons that form a single covalent bond. In structural formulas a single bond is represented by a line between bonded atoms. 83 18 ratings for this solution.
Although this form of covalent bond has a smaller density and is weaker than a double and triple bond it is the most stable. As a Lewis structure a single bond is denoted as AːA or A-A for which A represents an element. As examplesC-H is a carbon bonded to a hydrogen by a single covalent bond.
So when we ask for bond order we are wanting to know about what type of covalent bond is present. Bond energy The covalent bonds are flexible. A single bond is formed when only one pair of the electron is shared between the two participating atoms.
We even sometimes ask for overall bond order in a molecule. Think about your result. 17 What is electronegativity.
In closely related compounds with bonds between the same kinds of atoms the bond with the highest bond order is both the shortest and the strongest. The overall bond order is all the bond orders added up to a grand total. For example in diatomic nitrogen NN the bond number is 3 in ethyne HCCH the bond number between the two carbon atoms is also 3 and the CH bond order is 1.
The directional bias of rotation for the first-generation autonomous single bond rotary motor 1a is 7129 with a chirality-matched fuel and hydrolysis catalyst. The number of pairs of electrons in a covalent bond equals the bond order. A Electronegativity increases when moves from left to right in the periodic tablein the period.
The resulting Lewis electron dot structure is. It is represented by one dash -. Atoms are bonded by a single covalent bond.
S-O is a sulfur bonded to an oxygen by. A chemical bond between two atoms where there. Oxygen is closest to the upper right-hand corner of the periodic table and.
Polarity depends on the difference in electronegativities of two atoms forming covalent bond. A single covalent bond involves the sharing of electrons. One pair of shared electrons is a single covalent bond.
A single straight line is used to represent a single covalent bond between atoms. The number of pairs of electrons in a covalent bond equals the bond order. Single bonds have a bond order of one and multiple bonds with bond orders of two a double bond and three a triple bond are quite common.
The electrons are attracted to the positively charged nuclei of the atoms. We even sometimes ask for overall bond order in a molecule. All of the bond are single bonds.
Covalent bonds are formed when two atoms begin sharing electrons. For the formation of this bond presence of an atom with single valency is required. Each hydrogen atom with its single electron will form a covalent bond with the oxygen atom where it has a single electron.
Its structural simplicity should. It is very straight forward - a single bond has a bond order of 1 a double bond has a bond order of 2 and a triple bond has a bond order of 3. The following diagram represents the structural formula of pentane C3H8.
The bond number itself is the number of electron pairs covalent bonds between two atoms. When two atoms share one electron pair between each other then they are said to be bonded by single covalent bond denoted by single dash joining the atoms. A nonpolar covalent compound involves the equal sharing of electrons.
The oxygen atom follows the octet rule with two pairs of. A covalent bond is the sharing of a pair of valence electrons by two atoms What is a single covalent bond or single bond A single covalent bond or single bond is the sharing of one pair of valence electrons. A covalent compound is made when two or more nonmetal atoms bond by sharing valence electrons.
So when we ask for bond order we are wanting to know about what type of covalent bond is present. Step 1 of 3. Ionic compounds are usually solids at room temperature.
In the first rendition each dot represents a shared electron and in the second rendition the bar represents both of the electrons shared in the single bond. A double covalent bond otherwise known as a double bond shares two pairs of electrons. Two shared pairs is a double covalent bond and three shared pairs is a triple covalent bond.

Oxidized Low Density Lipoproteins What Causes High Cholesterol Covalent Bonding Chemical Bond

Ib Chemistry Standard Level Notes Covalent Bonding

Four Covalent Bonds Carbon Has Four Valence Electrons And Here A Valence Of Four Each Hydrogen Atom Has One Vale Covalent Bonding Chemical Bond Ionic Bonding

Covalent Bonding Covalent Bond Bonds Between Two Nonmetals Electrons Are Shared Rather Than Transferred Electronegativities Are Not Strong Enough Ppt Download

Single Covalent Bond Definition And Examples

How Is Covalent Bond Is Formed A Plus Topper Formationofcovalentbond Covalent Bonding Bond Form Example

Single Covalent Bond Definition And Examples

Common Examples Of Covalent Compounds Covalent Bonding Chemistry For Kids Chemical Bond

Single Covalent Bond Definition And Examples

Covalent Compound Properties Molecular Geometry Higher Order Thinking Skills Higher Order Thinking

Covalent Bonds Biology For Majors I

How Is Covalent Bond Is Formed A Plus Topper Formationofoxygenmolecule Covalent Bonding Bond Form Example

Covalent Compound Properties Molecular Geometry Higher Order Thinking Skills Higher Order Thinking

Covalent Bond Practice Test Quiz Questions Proprofs Quiz

Covalent Bond Definition Properties Examples Facts Britannica

Single And Multiple Covalent Bonds Article Khan Academy

Notes 5 3 Covalent Bonds Covalent Bond A Force That Bonds Two Atoms Together By A Sharing Of Electrons Each Pair Of Shared Electrons Creates Ppt Download
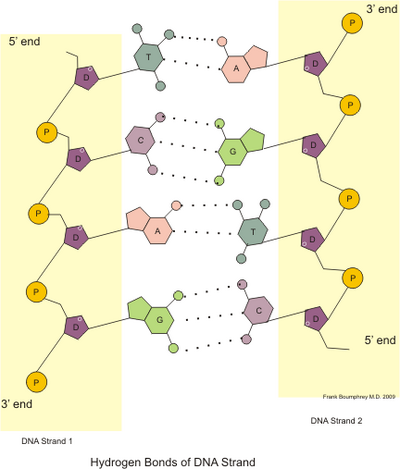
3 3 3 Outline How Dna Nucleotides Are Linked Together By Covalent Bonds Into A Single Strand Drugs Covalent Bonding Predictions

Notes 5 3 Covalent Bonds Covalent Bond A Force That Bonds Two Atoms Together By A Sharing Of Electrons Each Pair Of Shared Electrons Creates Ppt Download
Comments
Post a Comment